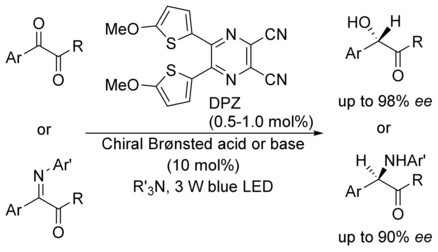
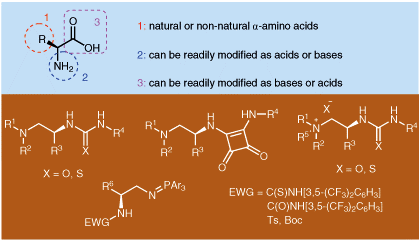
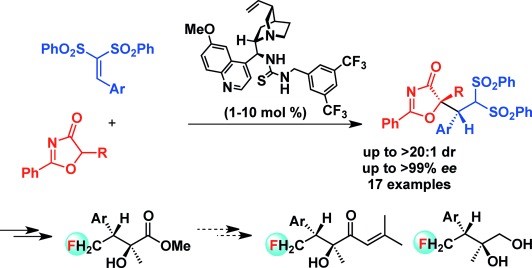
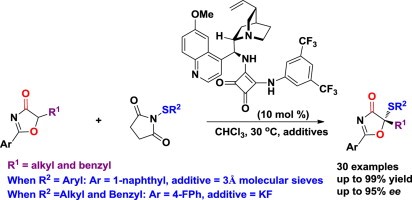
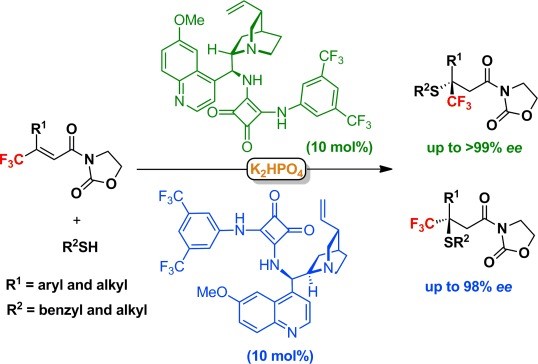
69. Li, L., Bai, X., Ye, X., Zhao, W., Tan, C.-H. Jiang, Z.* Organocatalytic Enantioselective Protonation for Photoreduction of Activated Ketones and Ketimines Induced by Visible Light. Angew. Chem. Int. Ed. 2017, 56, 13842−13846. (Highlighted by Synfacts 2017, 13, 1318, Contributors: Benjamin List, Grigory A. Shevchenko)
https://doi.org/10.1002/anie.201707899
68. Hong, S.-N., Liu, Y., Lee, R., Jiang, Z.* Asymmetric Tandem Conjugate Addition−Protonation to Forge Chiral Secondary C−O Bonds for Quaternary Carbon Stereocenters at the Nonadjacent β-Position. Chem. Commun. 2017, 53, 7493−7496.
https://doi.org/10.1039/C7CC03700G
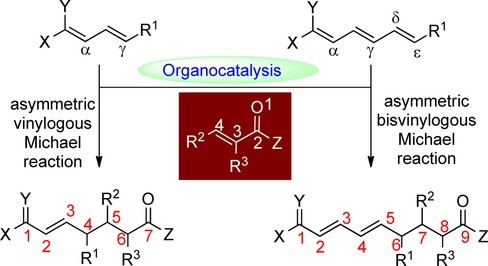
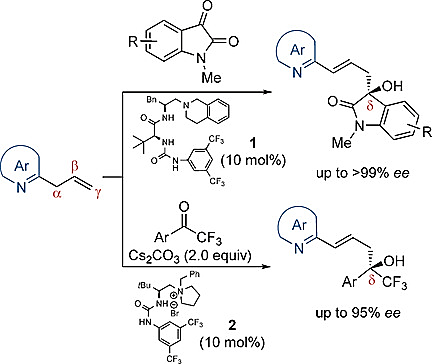
66. Bai, X., Zeng, G., Shao, T., Jiang, Z.* Catalytic Enantioselective γ-Selective Additions of 2-Allylazaarenes to Activated Ketones. Angew. Chem. Int. Ed. 2017, 56, 3684−3688. (Highlighted by Synfacts 2017, 13, 0544, Contributors: Benjamin List, Sebastian Schwengers)
https://doi.org/10.1002/anie.201700190
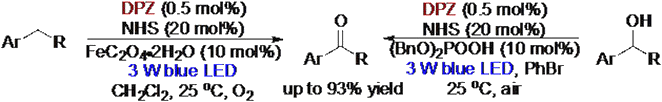
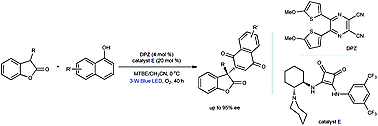
65. Liu, X., Lin, L., Ye, X., Tan, C.-H., Jiang Z.* Aerobic Oxidation of Benzylic sp3 C−H Bond through Cooperative Visible Light Photoredox Catalysis of N-Hydroxyimide and Dicyanopyrazine. Asian J. Org. Chem. 2017, 6, 422−425. (Invited)
https://doi.org/10.1002/ajoc.201600426
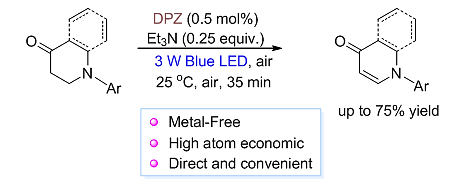
64. Shao, T., Jiang, Z.* Visible Light Mediated Photocatalytic Aerobic Dehydrogenation: A General and Direct Approach to Access 2,3-Dihydro-4-Pyridones and 4-Quinolones. Acta Chim. Sinica, 2017, 75, 70−73. (Invited)
http://sioc-journal.cn/Jwk_hxxb/CN/10.6023/A16080407
2016
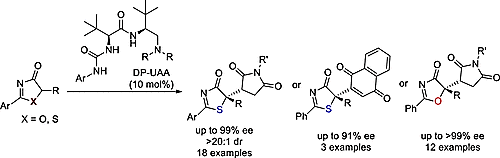
63. Li, J., Qiu, S., Ye, X., Zhu, B., Liu, H., Jiang Z.* Dipeptide-Based Chiral Tertiary Amine-Catalyzed Asymmetric Conjugate Addition Reactions of 5H-Thiazol/Oxazol-4-Ones. J. Org. Chem. 2016, 81, 11916−11923.
https://doi.org/10.1021/acs.joc.6b02384
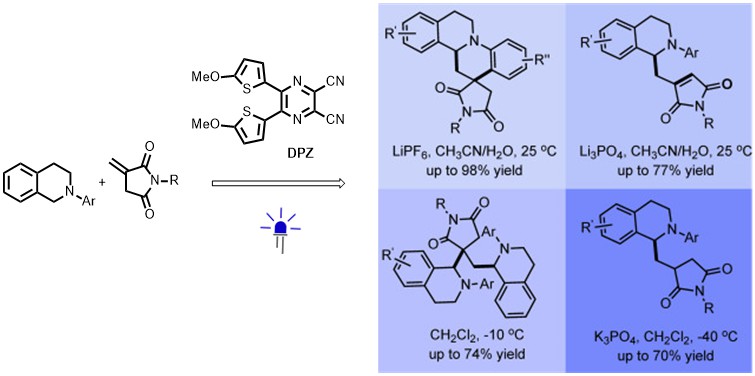
62. Liu, Y., Li, J., Ye, X., Zhao, X., Jiang Z.* Organocatalytic Asymmetric Formal Arylation of Benzofuran-2(3H)-ones and Cooperative with Visible Light Photocatalysis. Chem. Commun. 2016, 52, 13955−13958. (Selected as the cover picture)
https://doi.org/10.1039/C6CC07105H
61. Qiu, S., Tan, C.-H.,* Jiang Z.* Highly Chemo-, Enantio-, and Diastereo-selective [4+2] Cycloaddition of 5H-thiazol-4-ones with N-Itaconimides. Beilstein J. Org. Chem. 2016, 12, 2293−2297. (Invited)
https://doi.org/10.3762/bjoc.12.222
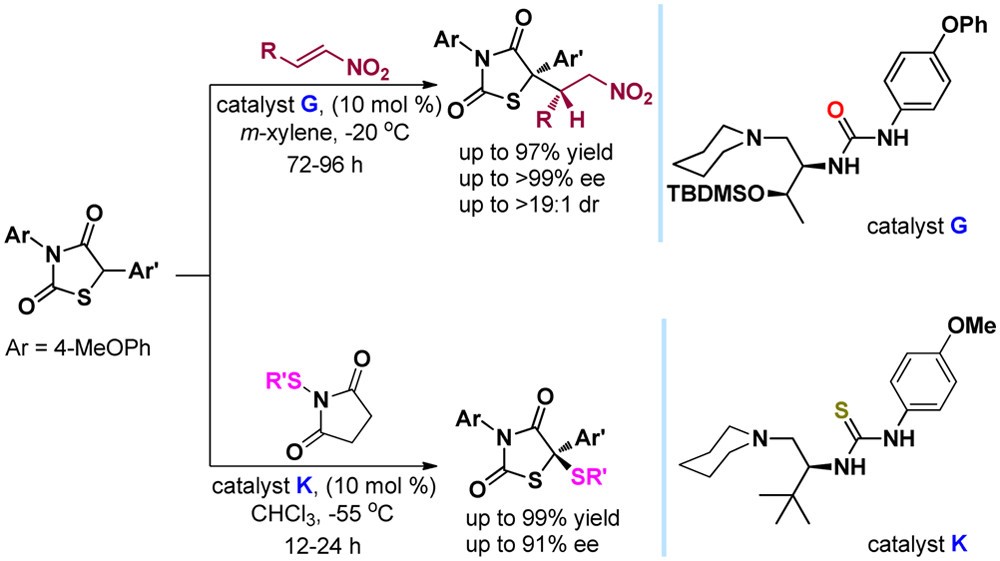
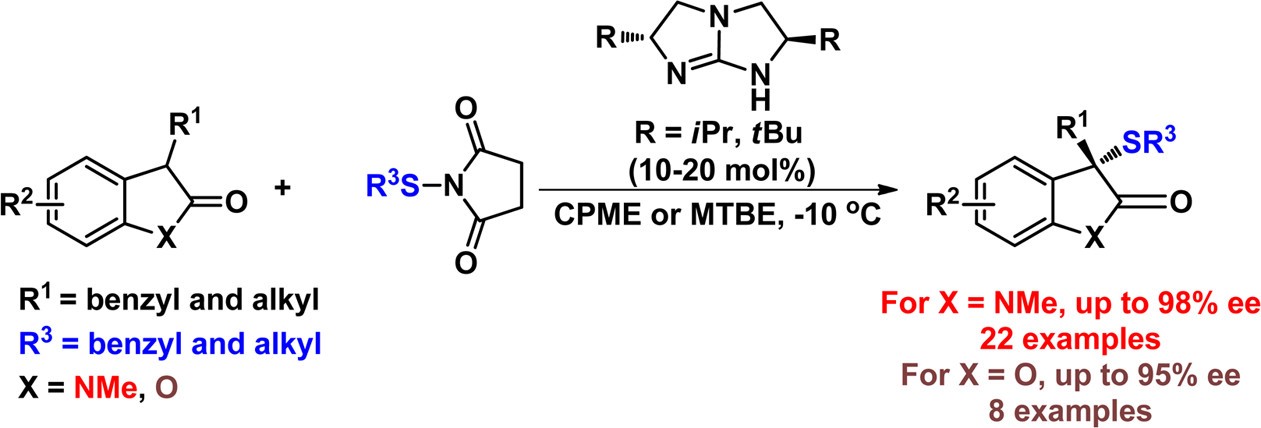
60. Jiao, L., Bu, L., Ye, X., Zhao, X., Jiang, Z.* Catalytic Asymmetric Addition and Sulfenylation of Diarylthiazolidin-2,4-Diones. J. Org. Chem. 2016, 81, 9620−9629.
https://doi.org/10.1021/acs.joc.6b01637
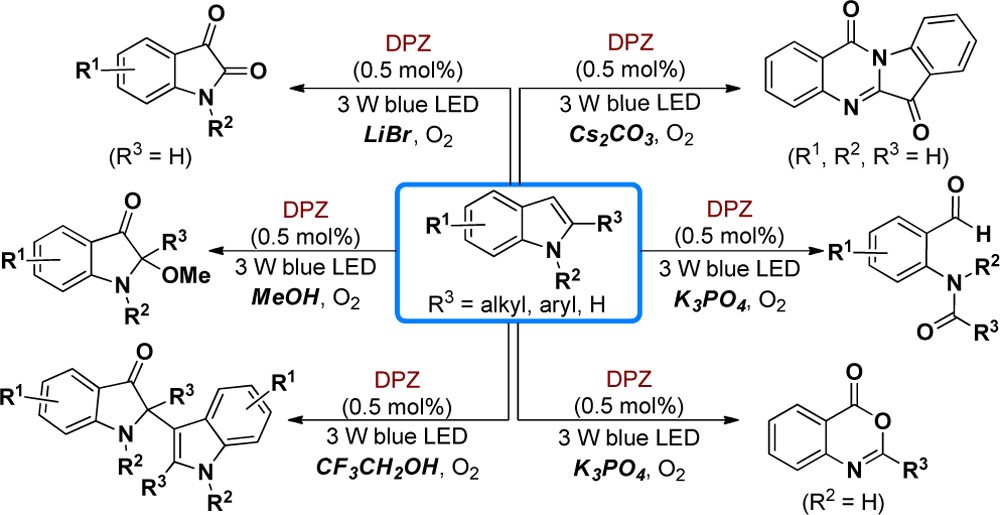
59. Zhang, C., Li, S., Bureš, F., Lee, R., Ye, X., Jiang, Z.* Visible Light Photocatalytic Aerobic Oxygenation of Indoles and pH as a Chemoselective Switch. ACS Catal. 2016, 6, 6853−6860.
https://doi.org/10.1021/acscatal.6b01969
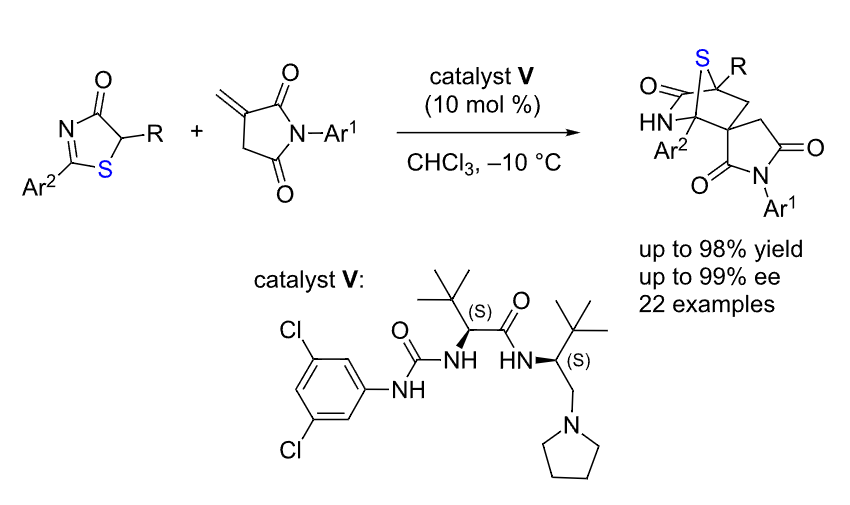
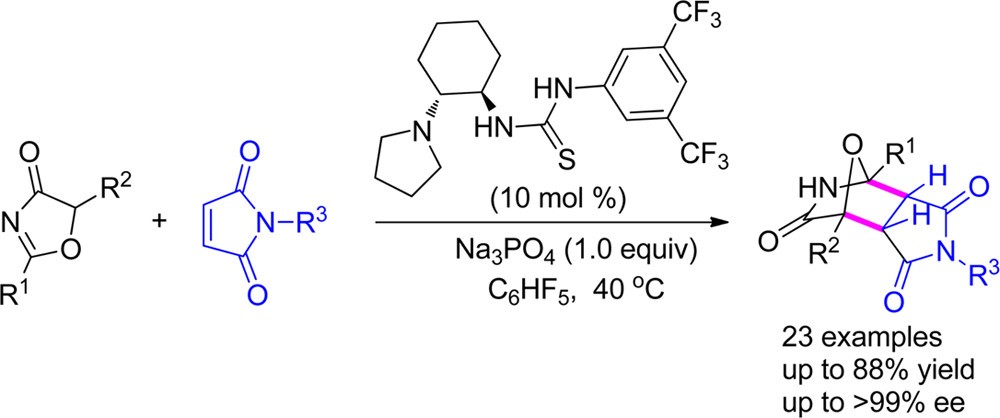
58. Qiu, S., Lee, R., Zhu, B., Coote, M. L., Zhao, X.,* Jiang, Z.* Highly Enantio- and Diastereoselective [4+2] cycloaddition of 5H-Oxazol-4-ones with N-Maleimides. J. Org. Chem. 2016, 81, 8061−8069.
https://doi.org/10.1021/acs.joc.6b01451
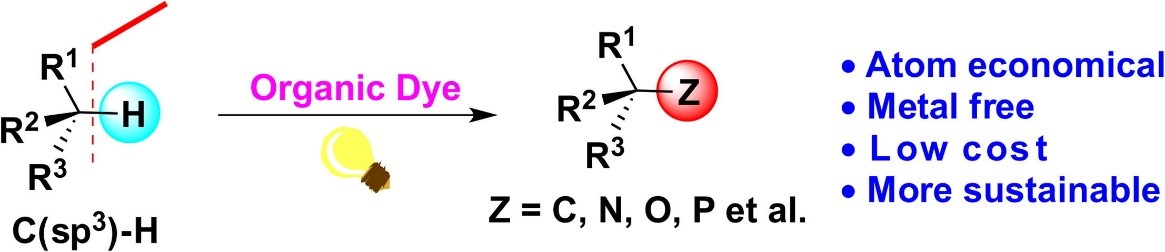
57. Wei, G., Basheer, C., Tan, C.-H., Jiang, Z.* Visible Light Photocatalysis in Chemoselective Functionalization of C(sp3)−H Bonds Enabled by Organic Dyes. Tetrahedron Lett. 2016, 57, 3801−3809. (Invited Digest)
https://doi.org/10.1016/j.tetlet.2016.07.032
56. Zhu, B., Qiu, S., Li, J., Coote, M. L., Lee, R., Jiang, Z.* Asymmetric [4+2] Annulation of 5H-Thiazol-4-Ones with a Chiral Dipeptide-Based Brønsted Base Catalyst. Chem. Sci. 2016, 6, 6060−6067.
https://doi.org/10.1039/C6SC02039A
55. Hou, X., Jing, Z., Bai, X., Jiang, Z.* A One-Pot Tandem Strategy in Catalytic Asymmetric Vinylogous Aldol Reaction of Homoallylic Alcohols. Molecules 2016, 21, 842.
https://doi.org/10.3390/molecules21070842
54. Wei, G., Zhang, C., Burěs, F., Ye, X., Tan, C.-H., Jiang, Z.* Enantioselective Aerobic Oxidative C(sp3)−H Olefination of Amines via Cooperative Photoredox and Asymmetric Catalysis. ACS Catal. 2016, 6, 3708−3712.
https://doi.org/10.1021/acscatal.6b00846
53. Jiao, L., Zhao, X., Liu, H., Ye, X., Li, Y., Jiang, Z.* Organocatalytic Asymmetric Conjugate Addition of Diaryloxazolidin-2,4-diones to Nitroolefins: An Efficient Approach to Chiral α-Aryl-α-Hydroxy Carboxylic Acids. Org. Chem. Front. 2016, 3, 470−474.
https://doi.org/10.1039/C5QO00428D
52. Jing, Z., Bai, X., Chen, W., Zhang, G., Zhu, B., Jiang, Z.* Organocatalytic Enantioselective Vinylogous Aldol Reaction of Allyl Ketones to Activated Acyclic Ketones. Org. Lett. 2016, 18, 260−263.
https://doi.org/10.1021/acs.orglett.5b03412
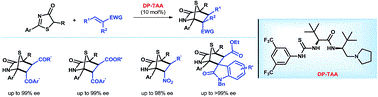
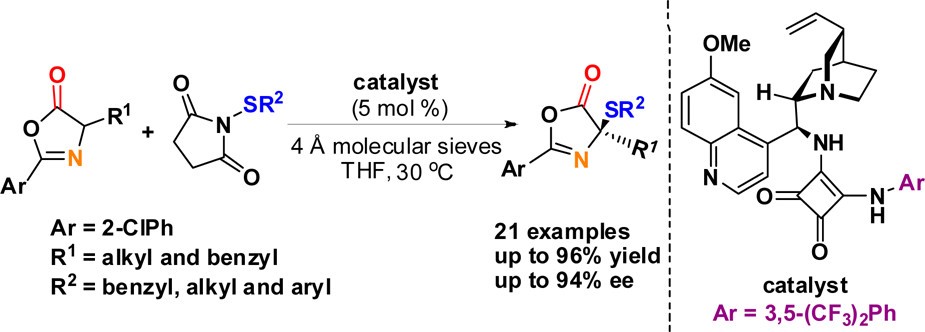
51. Zhu, B., Lee, R., Li, J., Ye, X., Hong, S.-N., Qiu, S., Coote, M. L., Jiang, Z.* Turning Chemoselective Switch in Asymmetric Organocatalysis of 5H-oxazol-4-ones and N-Itaconimides towards Tandem Conjugate Addition−Protonation or [4+2] Cycloaddition, Angew. Chem. Int. Ed. 2016, 55, 1299−1303.
https://doi.org/10.1002/anie.201507796
2015
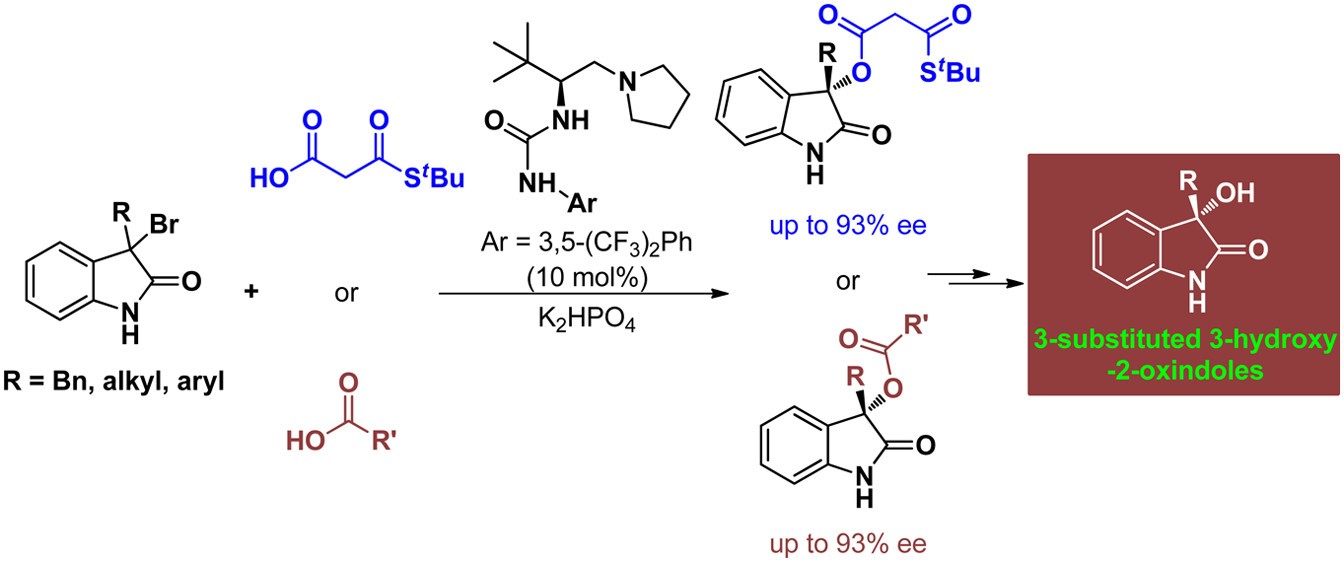
50. Bai, X., Jing, Z., Liu, Q., Ye, X., Zhang, G., Zhao, X., Jiang, Z.* L-Amino Acid-Based Urea−Tertiary Amine-Catalyzed Chemoselective and Asymmetric Stereoablative Carboxylation of 3-Bromooxindoles with Malonic Acid Half Thioesters, J. Org. Chem. 2015, 80, 12686−12696.
https://doi.org/10.1021/acs.joc.5b02286
49. Huang, L., Li, J., Zhao, Y., Ye, X., Liu, Y., Yan, L., Tan, C.-H., Liu, H., Jiang, Z.* Chiral Bicyclic Guanidine-Catalyzed Enantioselective Sulfenylation of Oxindoles and Benzofuran-2(3H)-ones. J. Org. Chem. 2015, 80, 8933−8941.
https://doi.org/10.1021/acs.joc.5b01606
48. Liu, X., Ye, X., Bureš, Liu, H., Jiang, Z.* Controllable Chemoselectivity in Visible-Light Photoredox Catalysis: Four Diverse Aerobic Radical Cascade Reactions, Angew. Chem. Int. Ed. 2015, 54, 11443−11447.
https://doi.org/10.1002/anie.201505193
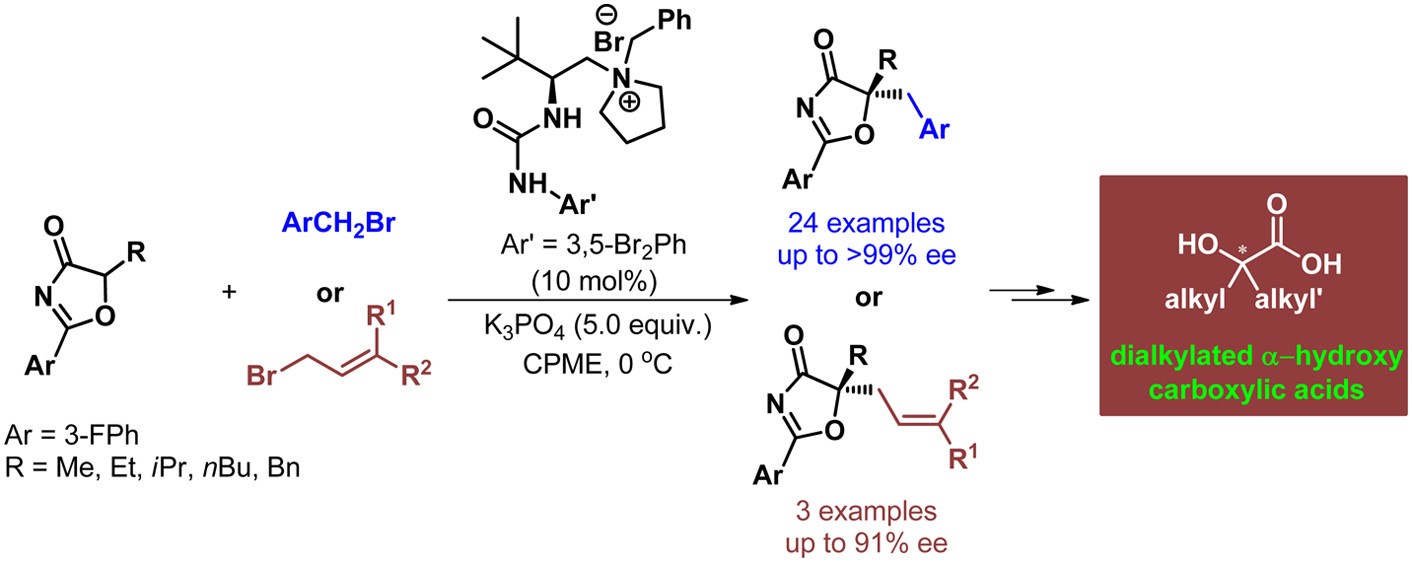
47. Duan, S., Li, S., Ye, X., Du, N.-N., Tan, C.-H. Jiang, Z. Enantioselective Synthesis of Dialkylated α-Hydroxy Carboxylic Acids through Asymmetric Phase-Transfer Catalysis, J. Org. Chem. 2015, 80, 7770−7778.
https://doi.org/10.1021/acs.joc.5b01081
46. Zhao, X., Zhu, B., Jiang, Z.* Acyclic Amino Acid-Based Bifunctional Chiral Tertiary Amines, Quaternary Ammoniums and Iminophosphoranes as Organocatalysts, Synlett, 2015, 26, 2216−2230. (Invited Account)
http://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0034-1378865
2014
44. Liu, Q., Qiao, B., Chin, K. F., Tan, C.-H., Jiang, Z.* Asymmetric Michael Addition of 5H-Oxazol-4-ones to Vinyl Sulfones: Highly Stereoselective Synthesis of Monofluorinated Analogs of 2-Tertiary Hydroxyl-3-Methyl-Substituted Carboxylic Acid Derivatives, Adv. Synth. Catal. 2014, 356, 3777−3783.
https://doi.org/10.1002/adsc.201400649
43. Xu, M., Qiao, B., Duan, S., Liu, H.,* Jiang, Z.* Highly Enantioselective α-Sulfenylation of 5H-Oxazol-4-Ones to N-(Sulfanyl)Succinimides, Tetrahedron 2014, 70, 8696−8702.
https://doi.org/10.1016/j.tet.2014.09.037
42. Qiao, B., Liu, X., Duan, S., Yan, L., Jiang, Z.* Highly Enantioselective Organocatalysis α-Sulfenylation of Azlactones. Org. Lett. 2014, 16, 672−675.
https://doi.org/10.1021/ol403303k
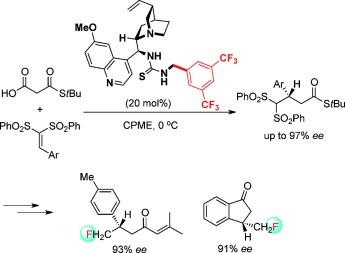
41. Chen, W., Jing, Z., Chin, K. F., Qiao, B., Zhao, Y., Yan, L., Tan, C.-H., Jiang, Z.* Catalytic Asymmetric Conjugate Addition of Mercaptans to β-Substituted-β-Trifluoromethyl Oxazolidinone Enoates: Access to Chiral Trifluoromethylated Tertiary Thioethers and Thiols, Adv. Synth. Catal. 2014, 356, 1292−1300.
https://doi.org/10.1002/adsc.201301027
40. Qiao, B., Liu, Q., Liu, H., Yan, L., Jiang, Z.* Asymmetric Decarboxylative 1,4-Addition of Malonic Acid Half Thioesters to Vinyl Sulfones: Highly Enantioselective Synthesis of 3-Monofluoromethyl-3-Arylpropanoic Esters, Chem. Asian. J. 2014, 9, 1252−1256.
https://doi.org/10.1002/asia.201400049
39. Jing, Z., Liu, J., Chin, K. F., Chen, W., Tan, C.-H., Jiang, Z.* Chiral Bicyclic Guanidine-Catalysed Conjugate Addition of α-Fluoro-β-Ketoesters to Cyclic Enones, Aust. J. Chem. 2014, 67, 1119−1123. (Invited)
https://doi.org/10.1071/CH14187
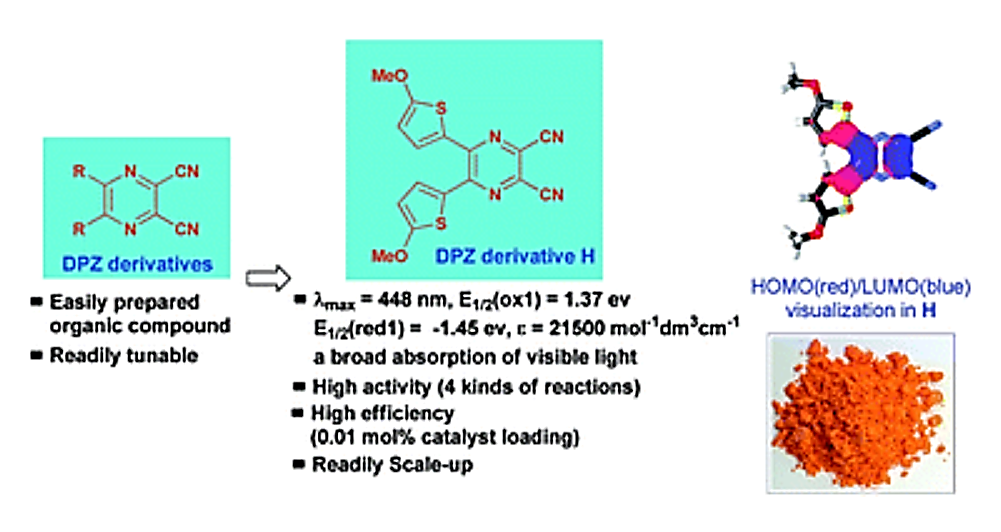
38. Zhao, Y.; Zhang, C.; Chin, K. F.; Pytela, O.; Wei, G.; Liu, H.; Bureš, F.;* Jiang, Z.* Dicyanopyrazine-Derived Push-Pull Chromophores for Highly Efficient Photoredox Catalysis, RSC Adv. 2014, 4, 30062−30067.
https://doi.org/10.1039/C4RA05525J