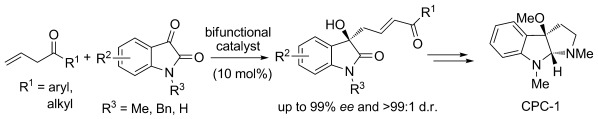
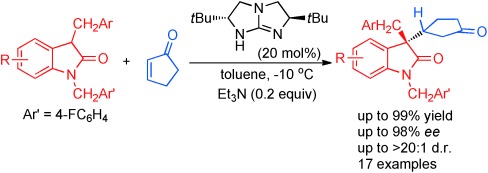
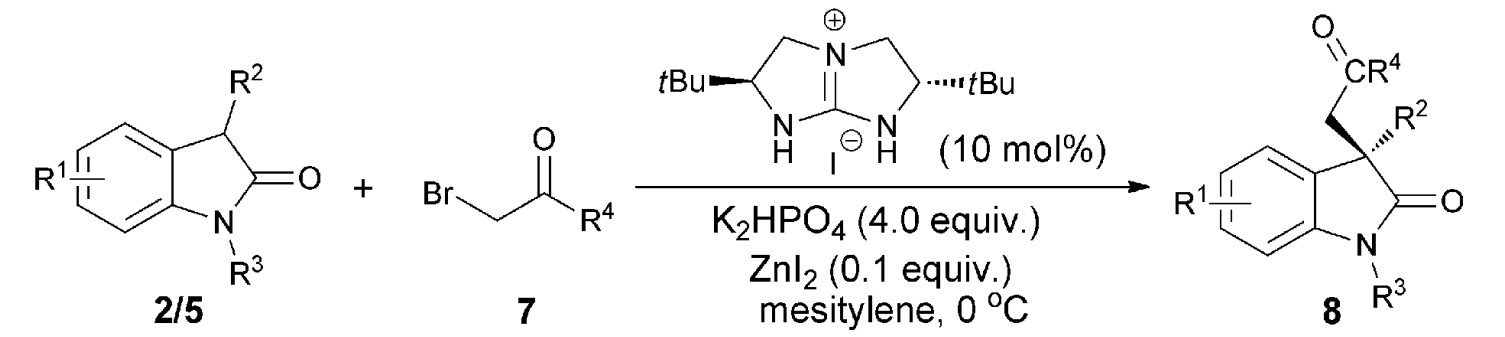
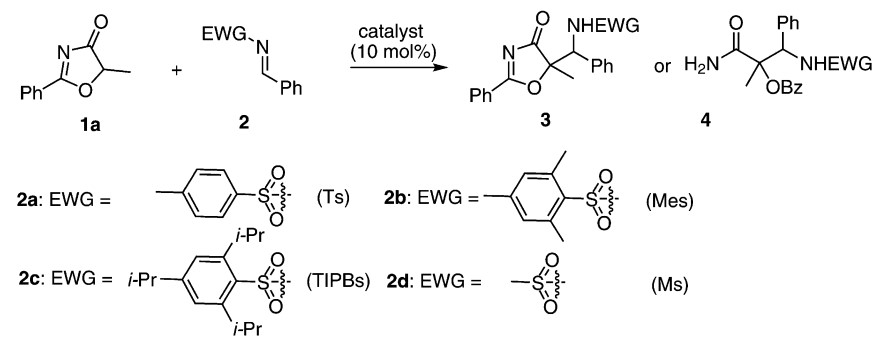
37. Zhu, B., Zhang, W., Lee, R., Han, Z., Yang, W., Tan, D., Huang, K.-W., Jiang, Z.* Direct Asymmetric Vinylogous Aldol Reaction of Allyl Ketones with Isatins: Divergent Synthesis of 3-Hydroxy-2-Oxindole Derivatives, Angew. Chem. Int. Ed. 2013, 52, 6666–6670 (Selected as the inside back cover picture of ACIE) (Highlighted by Synfacts 2013, 9, 0784, Contributors: Benjamin List, Mattia Riccardo Monaco)
https://doi.org/10.1002/anie.201302274
36. Yang, C., Chen, W., Yang, W., Zhu, B., Yan, L., Tan, C.-H., Jiang, Z.* Bicyclic-Guanidine-Catalyzed Asymmetric Michael Addition of 3-Substituted Oxindoles to 2-Cyclopentenone, Chem. Asian J. 2013, 8, 2960−2964.
https://doi.org/10.1002/asia.201301011
35. Chen, W., Yang, W., Yan, L., Tan, C.-H., Jiang, Z.* Bicyclic Guanidinium-Catalyzed Enantioselective Phase-Transfer Alkylation: Direct Access to Pyrroloindolines and Furoindolines, Chem. Commun. 2013, 49, 9854−9856.
https://doi.org/10.1039/C3CC46111D
34. Han, Z., Yang, W., Tan, C.-H., Jiang, Z.* Organocatalytic Asymmetric Mannich Reactions of 5H-Oxazol-4-ones: Highly Enantio- and Diastereoselective Synthesis of Chiral α-Alkylisoserine Derivatives, Adv. Synth. Catal. 2013, 355, 1505–1511.
https://doi.org/10.1002/adsc.201300135
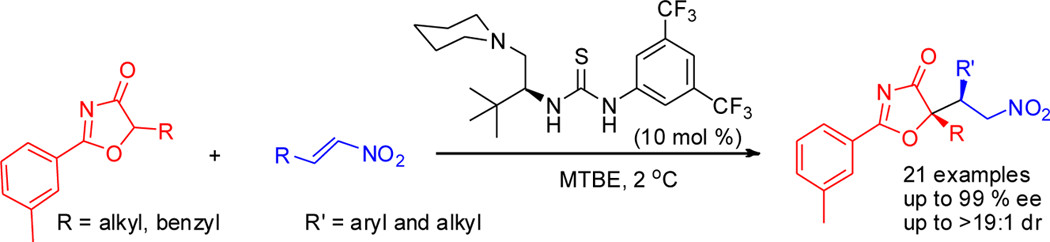
33. Qiao, B., An, Y., Liu, Q., Yang, W., Liu, H., Shen, J., Yan, L., Jiang, Z.* Organocatalytic Asymmetric Michael Addition of 5H-Oxazol-4-ones to Nitroolefins, Org. Lett. 2013, 15, 2358–2361.
https://doi.org/10.1021/ol401062z
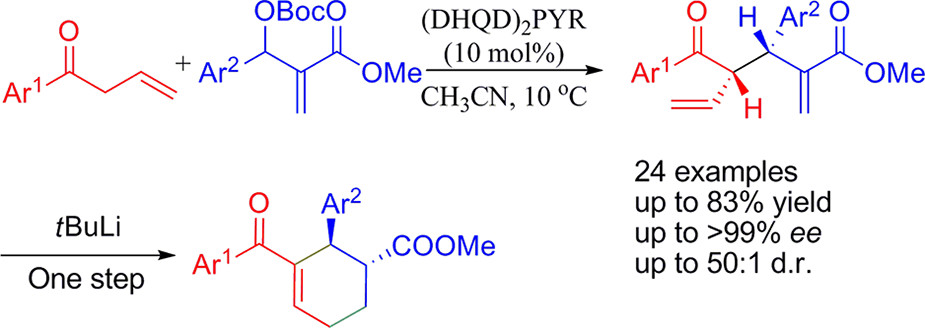
32. Tong, G., Zhu, B., Lee, R., Yang, W., Tan, D., Yang, C., Han, Z., Yan, L., Huang, K.-W., Jiang, Z.* Highly Enantio- and Diastereoselective Allylic Alkylation of Morita-Baylis-Hillman Carbonates with Allyl Ketones, J. Org. Chem. 2013, 78, 5067–5072.
https://doi.org/10.1021/jo400496z
31. Yan, L., Han, Z., Zhu, B., Yang, C., Tan, C.-H., Jiang, Z.* Asymmetric Alylic Alkylation of Morita-Baylis-Hillman Carbonates with α-Fluoro-β-Keto Esters, Beilstein J. Org. Chem. 2013, 9, 1853–1857.
https://www.beilstein-journals.org/bjoc/articles/9/216
30. Yan, L., Wu, X., Liu, H., Xie, L., Jiang, Z.* Catalytic Asymmetric Synthesis of γ-Butenolides by Direct Vinylogous Reactions, Mini-Rev. Med. Chem. 2013, 13, 845–853. (Invited review)
https://www.eurekaselect.com/article/50506
2012
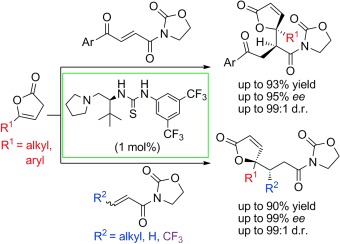
29. Zhang, W., Tan, D., Lee, R., Tong, G., Chen, W., Qi, B., Huang, K.-W., Tan, C.-H., Jiang, Z.* Highly Enantio- and Diastereoselective Reactions of γ-Substituted Butenolides via Direct Vinylogous Conjugate Additions, Angew. Chem. Int. Ed. 2012, 51, 69–73. (Highlighted by Synfacts 2012, 8, 1253, Contributors: Benjamin List, Ji Hye Kim)
https://doi.org/10.1002/anie.201205872
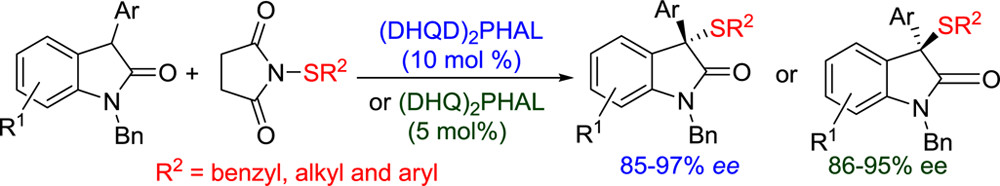
28. Han, Z., Chen, W., Dong, S., Yang, C., Liu, H., Pan, Y., Yan, L., Jiang, Z.* Highly Enantioselective Organocatalytic Sulfenylation of 3-Aryloxindoles, Org. Lett. 2012, 14, 4670–4673.
https://doi.org/10.1021/ol3021176
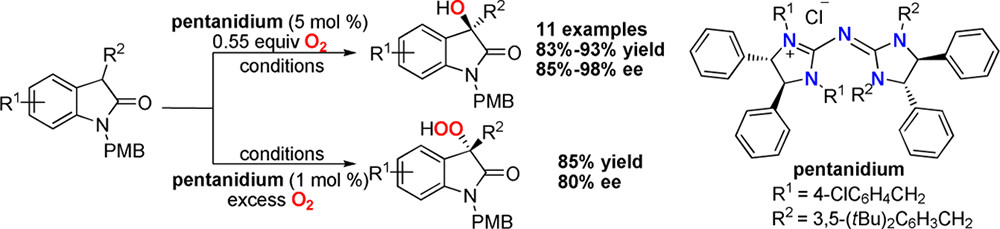
27. Yang, Y., Moinodeen, F., Chin, W., Ma, T., Jiang, Z., Tan, C.-H. Pentanidium–Catalyzed Enantioselective α-Hydroxylation of Oxindoles Using Molecular Oxygen, Org. Lett. 2012, 14, 4762–4765.
https://doi.org/10.1021/ol302030v
26. Yang, W., Tan, D., Li, L., Han, Z., Yan, L., Huang, K.-W., Tan, C.-H., Jiang, Z.* Direct Asymmetric Allylic Alkenylation of N−Itaconimides with Morita−Baylis−Hillman Carbonates, J. Org. Chem. 2012, 77, 6600-6607.
https://doi.org/10.1021/jo3012539
25. Li, L., Chen, W., Yang, W., Pan, Y., Liu, H., Tan, C.-H., Jiang, Z.* Bicyclic Guanidine-Catalyzed Asymmetric Michael Additions of 3-Benzyl-Substituted Oxindoles to N-Maleimides, Chem. Commun. 2012, 48, 5124-5126.
https://doi.org/10.1039/C2CC31587D
24. Yang, W., Tan, D., Lee, R., Li, L., Pan, Y., Huang, K.-W., Tan, C.-H., Jiang, Z.* Catalytic Diastereoselective Tandem Conjugate Addition-Ellimination Reaction of Morita-Baylis-Hillman C-Adducts via C-C Bond Cleavage, Chem. Asian J. 2012, 7, 771-777. (Top ten most accessed articles published in Chemistry-An Asian Journal in 2011 and 2012)
https://doi.org/10.1002/asia.201100863
23. Yan, L., Zhao, F., Gan, Y., Zhao, J., Jiang, Z.* Chemoselective Deprotection of Aryl tert-Butyldimethylsilyl Ethers Promoted by Phosphates, Synth. Commun. 2012, 42, 285-291.
https://doi.org/10.1080/00397911.2010.523859
Before 2011
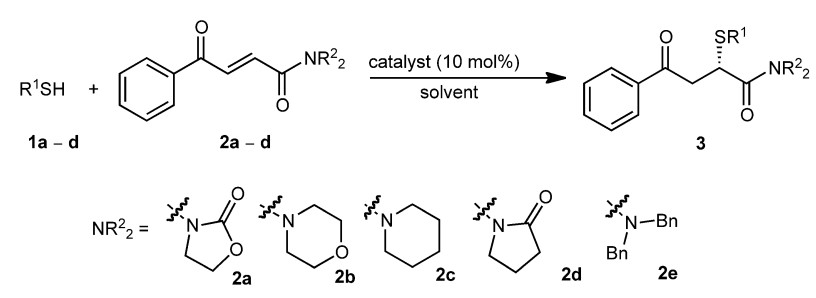
22. Zhao, F., Zhang, W., Yang, W., Pan, Y., Chen, W., Liu, H., Yan, L., Tan, C.-H., Jiang, Z.* Synthesis of Sulfur-Substituted α-Stereogenic Amides and Ketones: Highly Enantioselective Sulfa-Michael Additions of 1,4-Dicarbonyl But-2-enes, Adv. Synth. Catal. 2011, 353, 2624-2630.
https://doi.org/10.1002/adsc.201100227
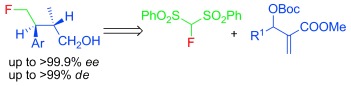
21. Yang, W., Wei, X., Pan, Y., Lee, R., Zhu, B., Liu, H., Yan, L., Huang, K.-W., Jiang, Z.,* Tan, C.-H.* Highly Enantio- and Diastereoselective Synthesis of β-Methyl-γ- Monofluoromethyl-Substituted Alcohols, Chem. Eur. J. 2011, 17, 8066-8070. (Very important, VIP paper)
https://doi.org/10.1002/chem.201100929
20. Zhu, B., Yan, L., Pan, Y., Lee, R., Liu, H., Han, Z., Huang, K.-W., Tan. C.-H.,* Jiang, Z.* Lewis Base Catalyzed Enantioselective Allylic Hydroxylation of Morita-Baylis-Hillman Carbonates with Water, J. Org. Chem. 2011, 76, 6894-6900.
https://pubs.acs.org/doi/10.1021/jo201096e
19. Zhao, Y., Pan, Y., Liu, H., Yang, Y., Jiang, Z.,* Tan, C.-H.* Fluorinated Aromatic Ketones as Nucleophiles in the Asymmetric Organocatalytic Formation of C-C and C-N bonds: a Facile Route to the Construction of Fluorinated Quaternary Stereogenic Centers, Chem. Eur. J. 2011, 17, 3571-3574.
https://doi.org/10.1002/chem.201003761
18. Yan, L., Yang, W., Li, L., Shen, Y., Jiang, Z.* A One-Pot Green Synthesis of Alkylidenesuccinimides, Chin. J. Chem. 2011, 29, 1906-1910.
https://doi.org/10.1002/cjoc.201180332
17. Pan, Y., Kee, C.-W., Jiang, Z., Ma, T., Zhao, Y., Yang, Y., Xue, H., Tan, C-.H.* Expanding the Utility of Brønsted Base Catalysis: Biomimetic Enantioselective Decarboxylative Reactions, Chem. Eur. J. 2011, 17, 8363‒8370.
https://doi.org/10.1002/chem.201100687
16. Wang, J., Liu, H., Fan, Y., Yang, Y., Jiang, Z.,* Tan, C.-H.* ‘Bicyclic Guanidine-Catalyzed Direct Asymmetric Allylic Addition of N-Aryl Alkylidene-Succinimides, Chem. Eur. J. 2010, 16, 12534-12537.
https://doi.org/10.1002/chem.201002183
15. Pan, Y., Zhao, Y., Ma, T., Yang, Y., Liu, H., Jiang, Z.,* Tan, C.-H.* Enantioselective Synthesis of α-Fluorinated β-Amino Acid Derivatives by an Aymmetric Mannich Reaction and Selective Deacylation/Decarboxylation Reactions, Chem. Eur. J. 2011, 16, 779-782.
https://doi.org/10.1002/chem.200902830
14. Jiang, Z., Pan, Y., Zhao, Y., Ma, T., Lee, R., Yang, Y., Huang, K.-W., Wong, M. W.,* Tan, C.-H.* Synthesis of Chiral Quaternary Carbon Center Bearing a Fluorine Atom: Enantio- and Diastereoselective Guanidine-Catalyzed Addition of Fluorocarbon Nucleophiles, Angew. Chem. Int. Ed. 2009, 48, 3627-3631. (Hot paper Chosen by Angewandte Chemie)
https://doi.org/10.1002/anie.200900964
13. Jiang, Z., Yang, Y., Pan, Y., Zhao, Y., Liu, H., Tan, C.-H.* Synthesis of α-Stereogenic Amides and Ketones via Enantioselective Conjugate Addition of 1,4-Dicarbonyl But-2-Enes, Chem. Eur. J. 2009, 15, 4925-4930.
https://doi.org/10.1002/chem.200802601
12. Jiang, Z., Ye, W., Yang, Y., Tan, C.-H.* Rate Acceleration of Triethylamine-Mediated Guanidine-Catalyzed Enantioselective Michael Reaction, Adv. Synth. Catal. 2008, 350, 2345-2351.
https://doi.org/10.1002/adsc.200800423
11. Ye, W., Jiang, Z., Zhao, Y., Goh, L. M. Serena, Leow, D., Soh, Y.-T., Tan, C.-H.* Chiral Bicyclic Guanidine as a Versatile Brønsted Base Catalyst for the Enantioselective Michael Reactions of Dithiomalonates and β-Keto Thioesters. Adv. Synth. Catal. 2007, 349, 2454-2458.
https://doi.org/10.1002/adsc.200700326
10. Jiang, Z., Zhang, Y., Ye, W., Tan, C.-H.* P-C Bond Formation via Direct and Three-Component Conjuagate Addition Catalyzed by 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) Tetrahedron Lett. 2007, 48, 51-54.
https://doi.org/10.1016/j.tetlet.2006.11.019
9. Jiang, Z., Chan, W. H.,* Lee, W. M. A. Synthesis of Enantiopure Sulfinimines (Thiooxime S-Oxides) Catalyzed by Yb(OTf)3 from p-Toluenesulfinamide and Aldehydes in Mild Reaction Conditions. J. Org. Chem. 2005, 70, 81-83.
8. Jiang, Z., Wang, Y.-G.* A Mild, Efficient and Selective Cleavage of Aryl tert-Butyldimethysilyl Ethers using KOH in Ethano, Chem. Lett. 2003, 32, 568-569.
7. Jiang, Z., Wang, Y.-G.* A Mild, Efficient and Selective Deprotection of t-Butylmethylsilyl-Protected Phenols Using Cesium Carbonate, Tetrahedron Lett. 2003, 44, 38593861.
6. Fu, X., Jiang, Z., Tan, C.-H.* Bicyclic Guanidine-Catalyzed Enantioselective Phospha-Michael Reaction: Synthesis of Chiral β-Aminophosphine Oxides and β-Aminophosphines, Chem. Commun. 2007, 5058-5060.
5. Cui, S.-L., Jiang, Z., Wang, Y.-G.* A General and Efficient Protocol for the Synthesis of Biaryl Ethers from Aryl Silyl Ethers using Cs2CO3, Synlett 2004, 10, 1829-1831.
4. Wang, Y.-G.,* Wu, X., Jiang, Z. A Mild and Efficient Selective Tetrahydropyranylation of Primary Alcohols and Deprotection of THP Ethers of Phenols and Alcohols using PdCl2(CH3CN)2 as Catalyst, Tetrahedron Lett. 2004, 45, 29732976.
3. Hua, J., Jiang, Z., Wang, Y.-G.* An Efficient and Chemoselective Deprotection of tert-Butyldimethylsilyl Protected Alcohols using SnCl2*2H2O as Catalyst, Chin. Chem. Lett. 2004, 15, 1430-1432.
2. Zhao, D., Ong, S.-W., Yue, Z., Jiang, Z., Toh, Y.-C., Khan, M., Shi, J., Tan, C.-H., Chen, J.,* Yu, H.* Dendrimer Hydrazides as Multivalent Transient Inter-cellular Linkers, Biomaterials 2008, 29, 3693-3702.
1. Xu, J., Fu, X., Low, R., Goh, Y.-P., Jiang, Z., Tan, C.-H.* Tandem Conjugate Addition-Elimination Reaction Promoted by Chiral Pyrrolidinyl Sulfonamides (CPS), Chem. Commun. 2008, 5526-5528.