2025
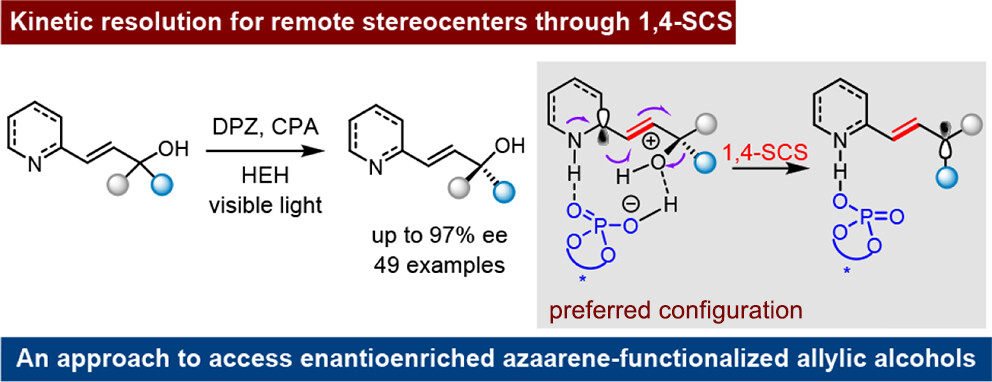
160. Bai, X.,# Yao, J.,# Zhang, M., Peng, Q., Li, W., Yin, Y., and Jiang, Z.,* Kinetic Resolution for 1,4-Spin-Center Shift-Based Reduction of Azaarene-Functionalized Secondary and Tertiary Allylic Alcohols. J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c15917. https://doi.org/10.1021/jacs.5c15917
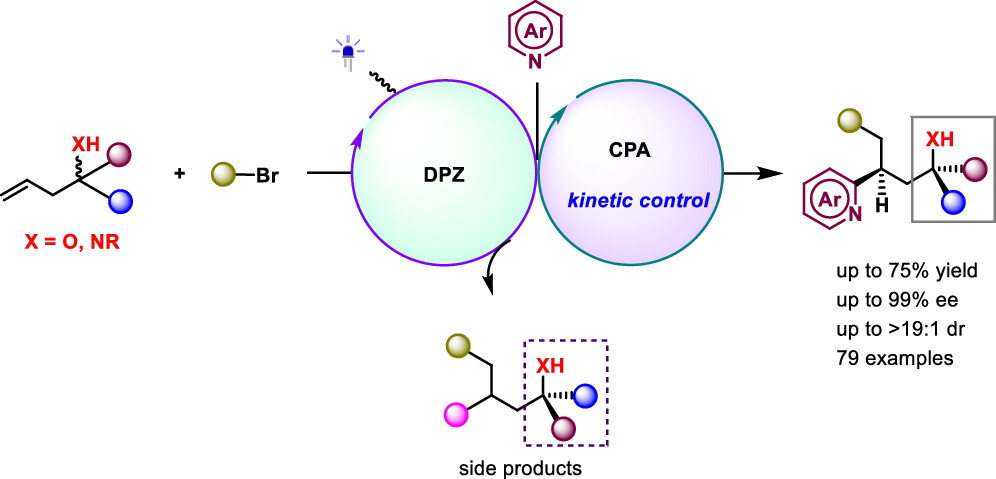
159. Shao, T., Li, Z., Nie, F., Li, Q., Zhao, X., and Jiang, Z.,* Leveraging electron donor–acceptor complexes for kinetic resolution in catalytic asymmetric photochemical synthesis. Nat. Chem. 2025, 17, 1722–1731. DOI:10.1038/s41557-025-01973-y. https://doi.org/10.1038/s41557-025-01973-y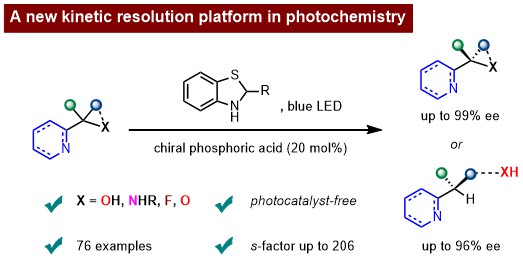
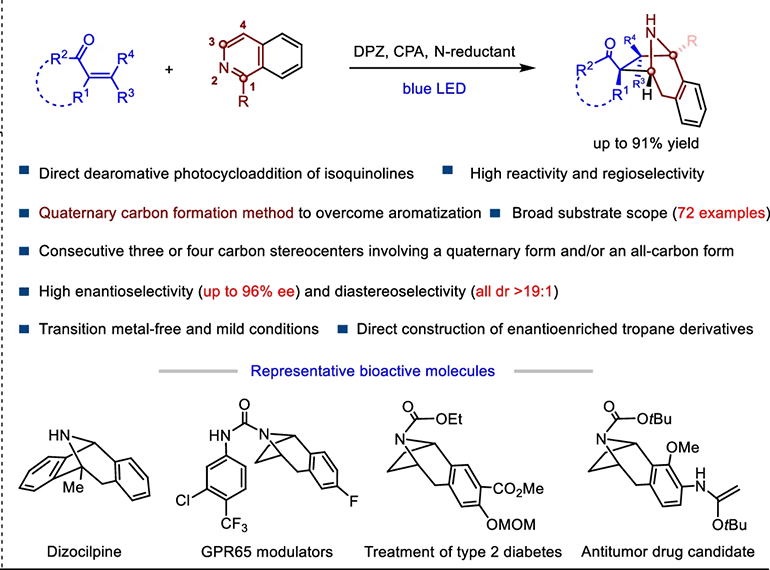
158. Huo, J., Yang, S., Kong, M., Xi, M., Qiao, B.,* and Jiang, Z.,* Photoredox catalytic asymmetric dearomative [3+2] cycloaddition of isoquinolines with enones. Nat. Commun. 2025, DOI: 10.1038/s41467-025-62876-7. https://doi.org/10.1038/s41467-025-62876-7
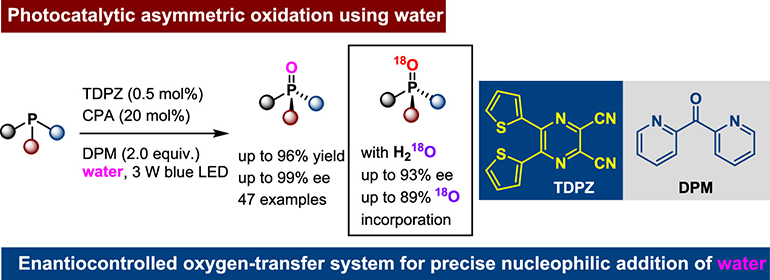
157. Dai,Y., Huang,Y., Wang,Y., Lin,Y., Liu,Y., Wei,Q., Zhao,X., Yin,Y.,* and Jiang,Z.,* Photocatalytic Asymmetric Oxidation of Phosphines with Water. J. Am. Chem. Soc. 2025, DOI:10.1021/jacs.5c11656.
https://doi.org/10.1021/jacs.5c11656
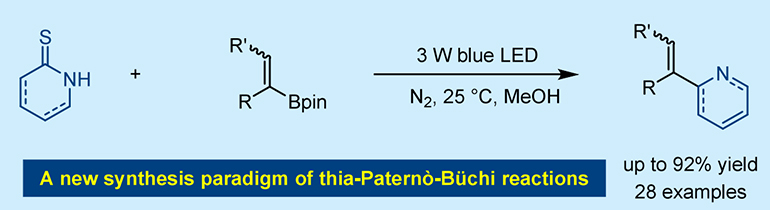
156. Lin, Y., Sun, X., Dai, Y., Liu, S., Yin, Y.,* and Jiang, Z.,* Photochemical Synthesis of α‑Substituted Vinylazaarenes Enabled by Thia-PaternÒ-BÜchi Reactions. Org. Lett. 2025, DOI:10.1021/acs.orglett.5c02390.
https://doi.org/10.1021/acs.orglett.5c02390
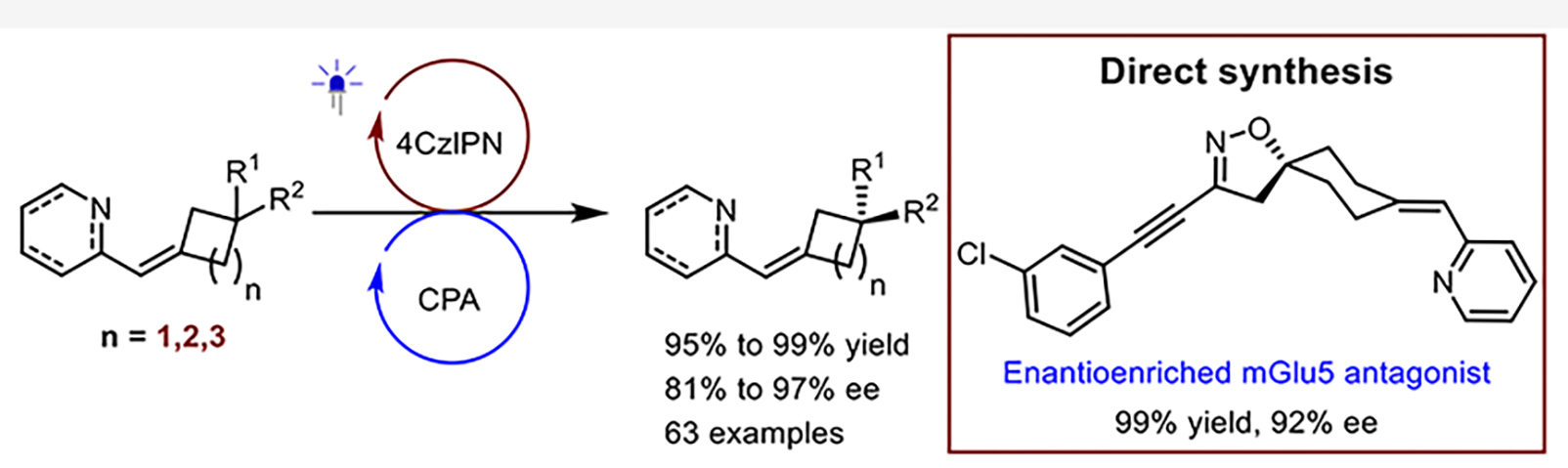
155. Zeng,G., Shi,W., Wang,Z., Zhao,X., Yin,Y.,* and Jiang,Z.,* Triplet Energy Transfer-Based Deracemization of Axially Chiral Alkenes Enabled by a Dual catalyst system. J. Am. Chem. Soc. 2025, DOI:10.1021/jacs.5c10123.
https://doi.org/10.1021/jacs.5c10123
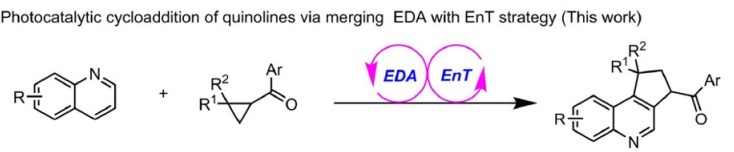
 154. Han,C.,# Wang, L.,# Zhao, X., Qiao, B.,* and Jiang,Z.,* Photocycloaddition of quinolines and cyclopropanes via electron donor-acceptor relay energy transfer strategy. Sci. China Chem. 2025, 68, DOI:10.1007/s11426-025-2650-x.https://doi.org/10.1007/s11426-025-2650-x
154. Han,C.,# Wang, L.,# Zhao, X., Qiao, B.,* and Jiang,Z.,* Photocycloaddition of quinolines and cyclopropanes via electron donor-acceptor relay energy transfer strategy. Sci. China Chem. 2025, 68, DOI:10.1007/s11426-025-2650-x.https://doi.org/10.1007/s11426-025-2650-x
153. Cao,S.,* Meng,Y., Guo,J., Chen,J., and Jiang,Z.,* In-Depth Insights into the Enantioselective Ketyl-Olefin Cyclizations Promoted by Reductive Multisite Proton-Coupled Electron Transfer. ACS Catal. 2025,15, 11852-11860. DOI: 10.1021/acscatal.5c02740. http://doi.org/10.1021/acscatal.5c02740
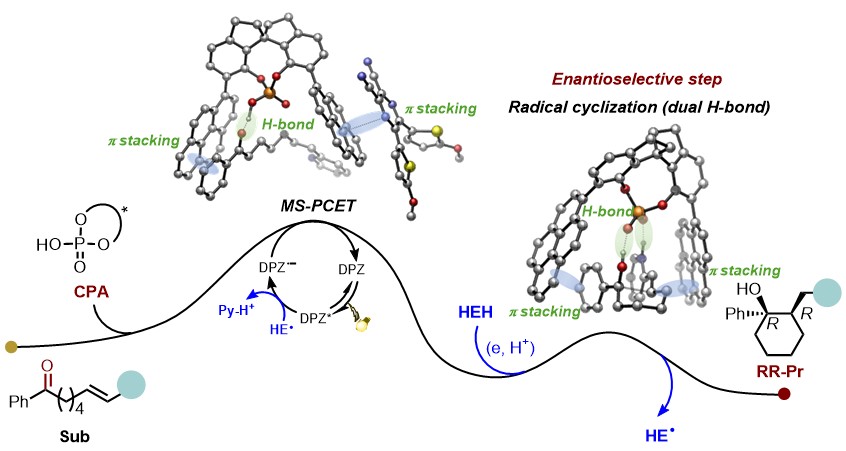
152. Liu,F., Guo,Y., Lu,W., Zhao,X., Yin,Y.,* and Jiang,Z.,* Precise Construction of Spiro Stereocenters via Enantioselective Radical Addition through Modulating Photocatalysis from Redox to Energy Transfer. Chem. Sci. 2025,16, 10555-10562. DOI: 10.1039/D5SC01583A.
http://doi.org/10.1039/D5SC01583A
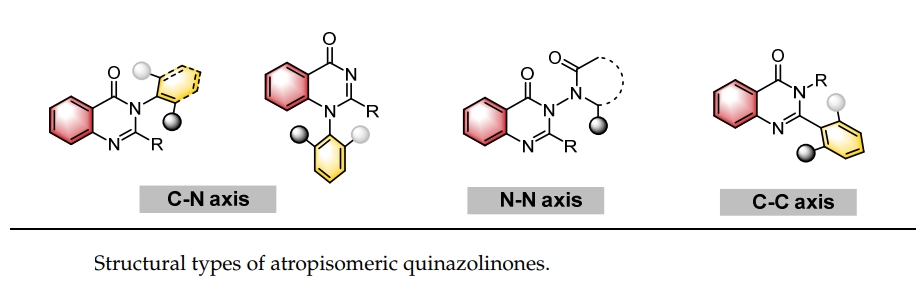
151. Liu, Y., Wang J., Yin, Y.,* and Jiang, Z.,* Recent Advances in Catalytic Atroposelective Synthesis of Axially Chiral Quinazolinones. Catalysts 2025, 15, 426-446. DOI: 10.3390/catal1505042.
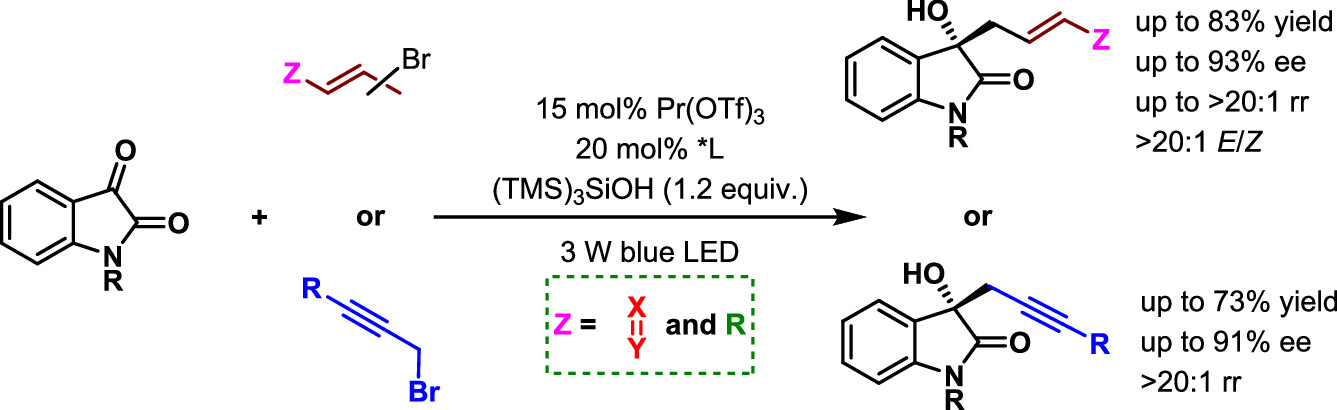
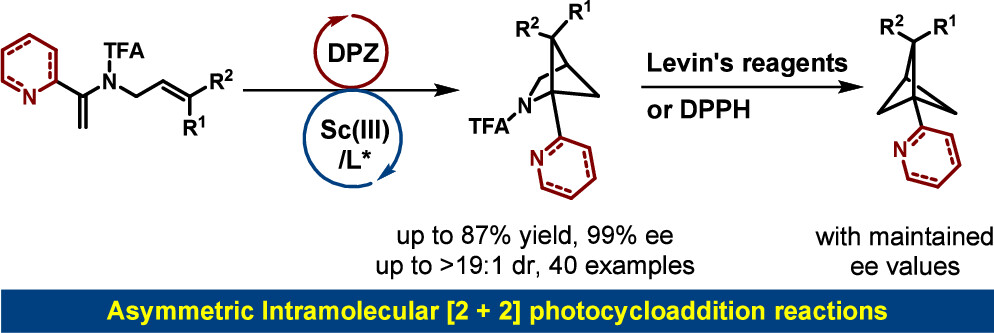
150. Dong, T.,# Pan, Y.,# Zhao, X., Yin, Y., and Jiang, Z.,* Chiral Lewis Acid-Catalyzed Intramolecular [2 + 2] Photocycloaddition: Enantioselective Synthesis of Azaarene Functionalized Azabicyclo[2.1.1]hexanes and Bicyclo[1.1.1]pentanes. J. Am. Chem. Soc. 2025, 147, 12410-12417. DOI: 10.1021/jacs.5c03542.
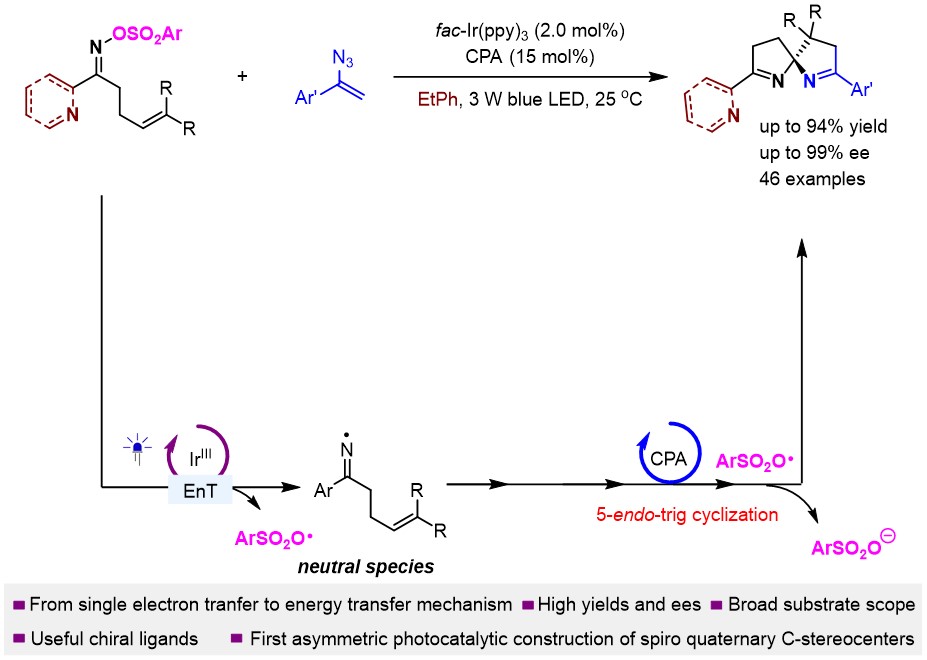
149. Shao, T., Nie, F.,Cao, S., Li, Q., Zhao, X., Yin, Y., and Jiang, Z.,* Kinetic Resolution of Racemic Radicals in Asymmetric Photoredox Minisci Reactions with Azaarenes for Precise Construction of Two Non-adjacent Stereocenters. J. Am. Chem. Soc. 2025, 147, 10002-10011. DOI: 10.1021/jacs.5c01623.
http://doi.org/10.1021/jacs.5c01623
148. Zhang, J.,‡ Guo, J.,‡ Xu, R., Zheng, D., Lian, K., Zhang, Z., Cao, S.,* and Jiang, Z., * Catalytic asymmetric photocycloaddition reactions mediated by enantioselective radical approaches. Chem. Sci. 2025, 16, 5957-5966. DOI: 10.1039/D5SC00358J.
147. Yin, Y., You, M., Li, X., Jiang, Z.* Catalytic asymmetric photocycloaddition reactions mediated by enantioselective radical approaches. Chem. Soc. Rev. 2025, 54, 2246-2274. DOI: 10.1039/d5cs00019j.
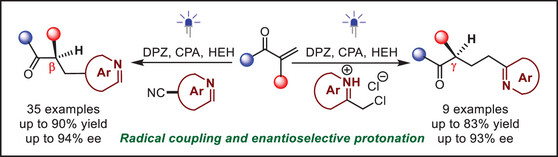
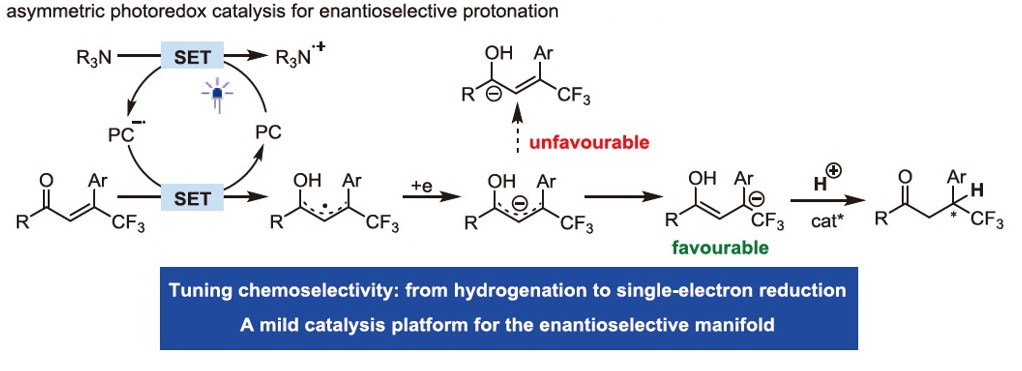
146. Sun, X., Zhu, W., Yin, Y., Zhao, X., Jiang, Z.* From Radical Coupling to Enantioselective Controlled Protonation: Advancing Precise Construction of Stereocenters. J. Am. Chem. Soc. 2025, 147, 4382-4392. DOI: 10.1021/jacs.4c15276.
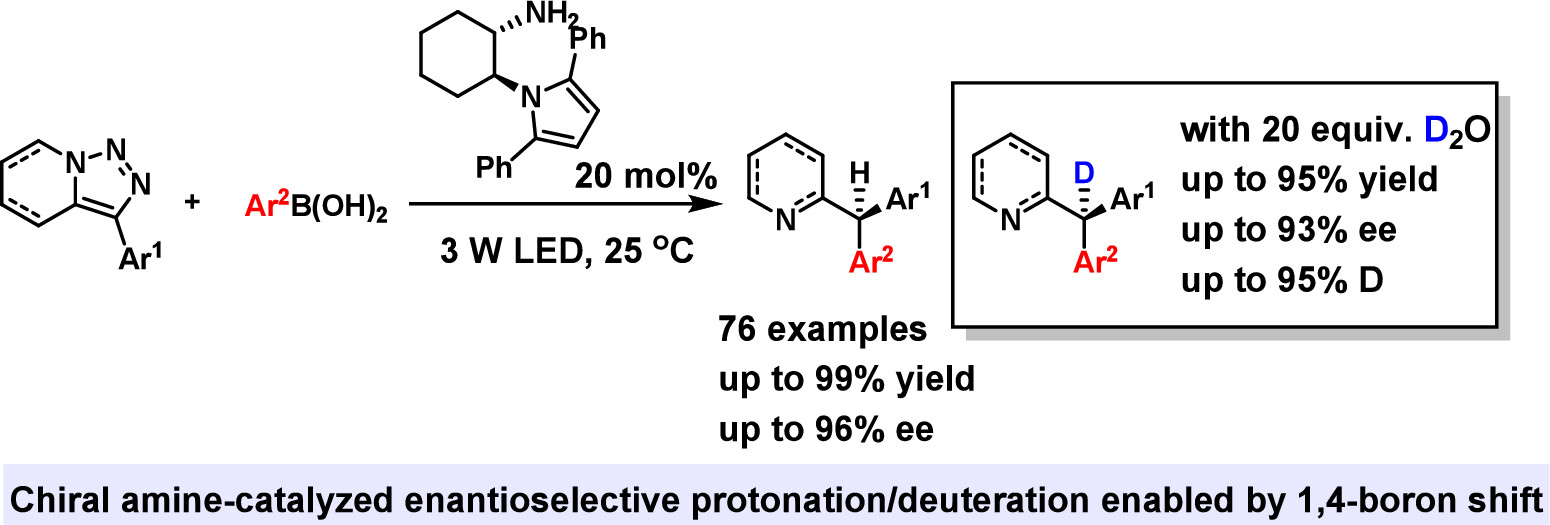
145. Jiang,C.,† Meng, Y.,† Huang, Y., Liu, C., Yin, Y., Zhao, X., Cao, S., *Jiang, Z.* Chiral Primary Amine-Catalyzed Asymmetric Photochemical Reactions of Pyridotriazoles with Boronic Acids to Access Triarylmethanes. J. Am. Chem. Soc. 2025, 147, 5320-5329. DOI: 10.1021/jacs.4c16811.
http://doi.org/10.1021/jacs.4c16811
144. Li, Q., Zhao, X.,Yin, Y., Shao, T., Jiang, Z.* Asymmetric Photoredox Catalytic Minisci-Type Reactions of α‑Bromide Amides. Org. Lett. 2025, 27, 1244-1249. DOI: 10.1021/acs.orglett.4c04791.
2024
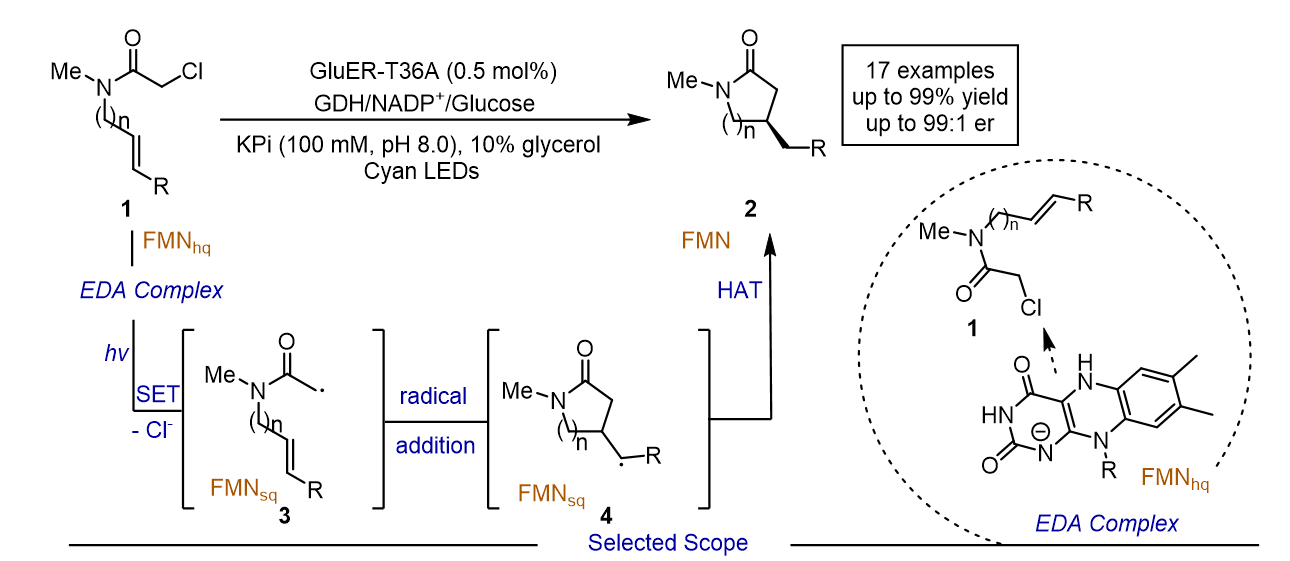
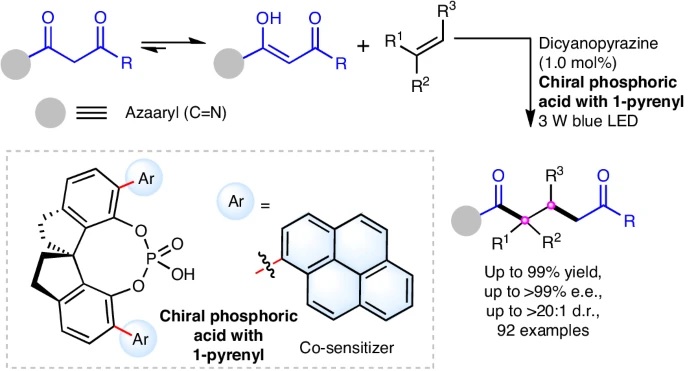
143. Guo, Y.-Y.*, Tian, Z.-H., Zhang, L., Han, Y.-C., Zhang, B.-B., Xing, Q., Shao, T., Liu, Y., Jiang, Z.*. J. Am. Chem. Soc. 2024,146, 31012-31020. DOI: 10.1021/jacs.4c10441.
142. Li, F., Liu, F.-Y., Zhao, X*, Yin, Y., Yu, B., Zhang, J.*, Jiang, Z.* Unified Enantioselective Allylations and Vinylogous Reactions Enabled by Visible Light-Driven Chiral Lewis Acid Catalysis. ACS Catal. 2024, 14, 16479−16487. DOI: 10.1021/acscatal.4c04638.
https://pubs.acs.org/doi/10.1021/acscatal.4c04638
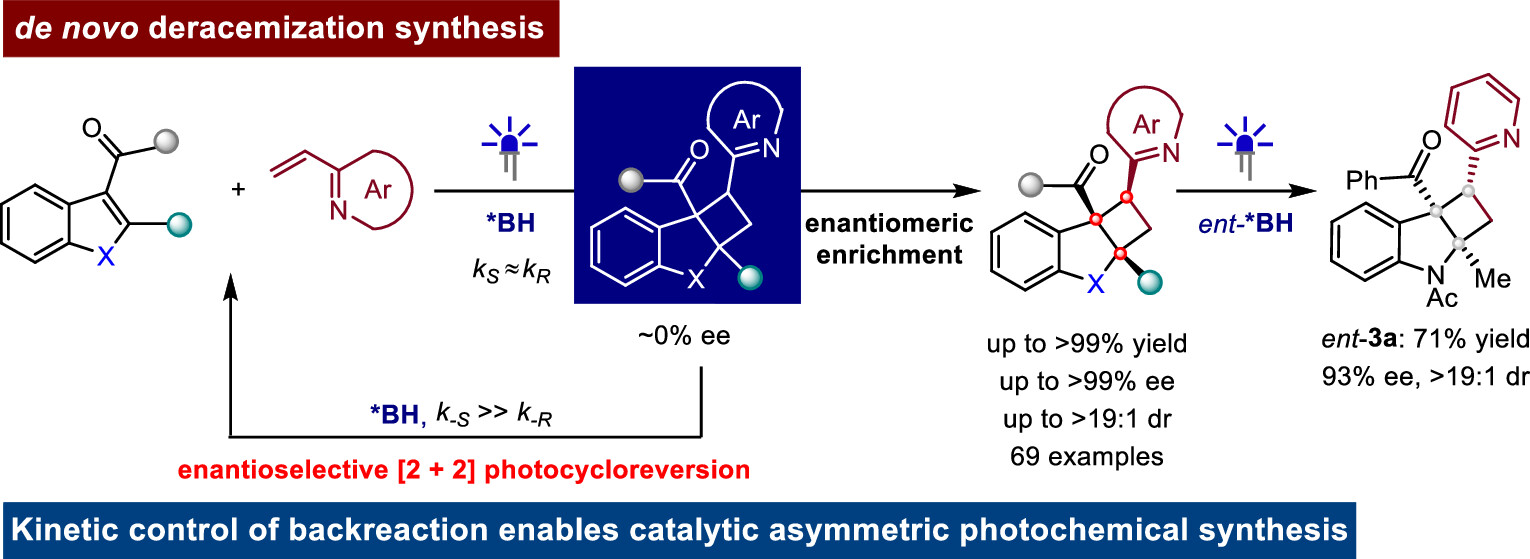
140. Wang, J., Fu, Q., Cao, S., Lv, X., Yin, Y., Ban, X., Zhao, X., Jiang, Z.* Enantioselective [2 + 2] Photocycloreversion Enables De Novo Deracemization Synthesis of Cyclobutanes. J. Am. Chem. Soc. 2024, 146, 22840−22849. DOI: 10.1021/jacs.4c08290.
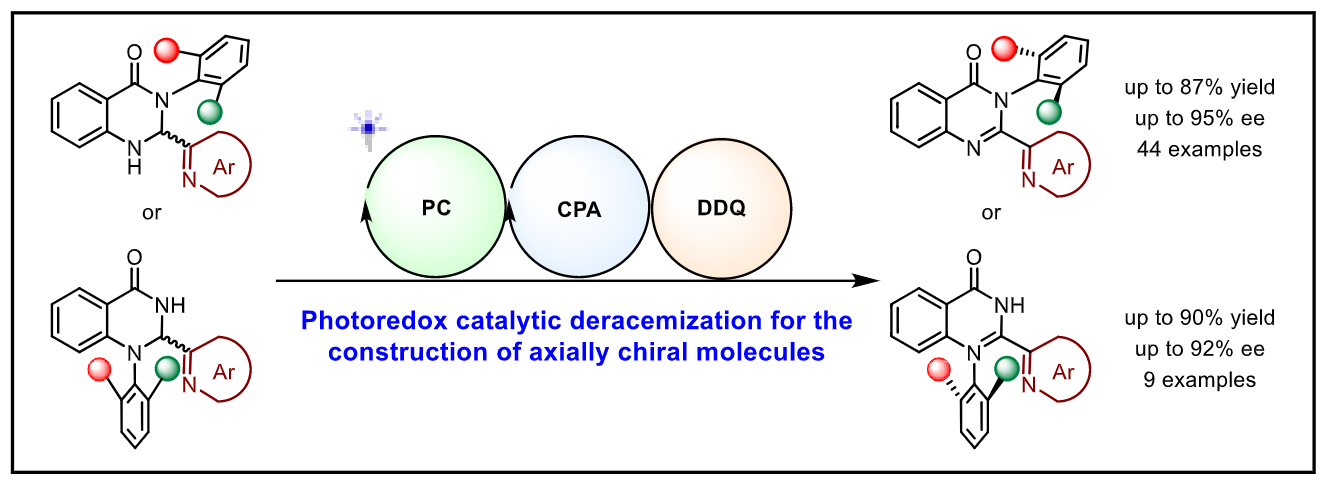
139. Liu, Y., Chu, M., Li, X., Cao, Z., Zhao, X., Yin, Y.*, Jiang, Z.* Photoredox Catalytic Deracemization Enabled Enantioselective and Modular Access to Axially Chiral N-Arylquinazolinones. Angew. Chem. Int. Ed. 2024, DOI: 10.1002/anie.202411236.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202411236
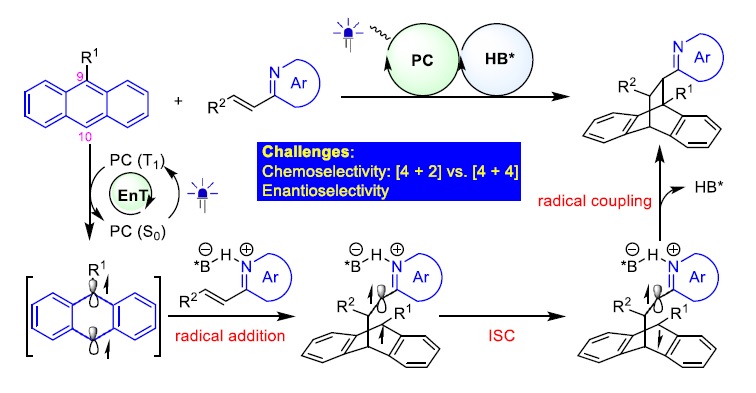
138. Tian, D., Shi, W., Sun, X., Zhao, X., Yin, Y., Jiang, Z.*, Catalytic asymmetric [4+2] dearomative photocycloadditions of anthracene and its derivatives with alkenylazaarenes. Nat. Commun. 2024, 15 (1), 4563.
https://doi.org/10.1038/s41467-024-48982-y
137. Sun, X., Liu, Y., Yin, Y., Ban, X., Zhao, X., Jiang, Z.*, Asymmetric Photoredox Catalytic Formal De Mayo Reaction Enabled by Sensitization-Initiated Electron transfer. Nat. Chem. 2024, 16, 1169-1176.
https://www.nature.com/articles/s41557-024-01502-3
136. Kong, M., Wang, Z., Ban, X., Zhao, X., Yin, Y., Zhang, J., Jiang, Z.*, Radical Cross Coupling and Enantioselective Protonation through Asymmetric Photoredox Catalysis. Adv. Sci. 2024, 11 (12), 2307773.
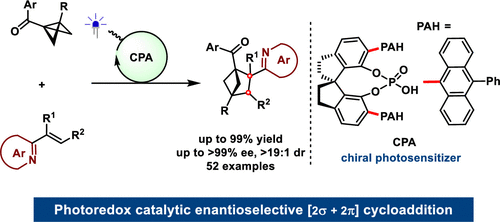
135. Fu, Q., Cao, S., Wang, J.*, Lv, X.*, Wang, H., Zhao, X., Jiang, Z.*, Enantioselective [2π + 2σ] Cycloadditions of Bicyclo[1.1.0]butanes with Vinylazaarenes through Asymmetric Photoredox Catalysis. J. Am. Chem. Soc. 2024, 146 (12), 8372-8380.
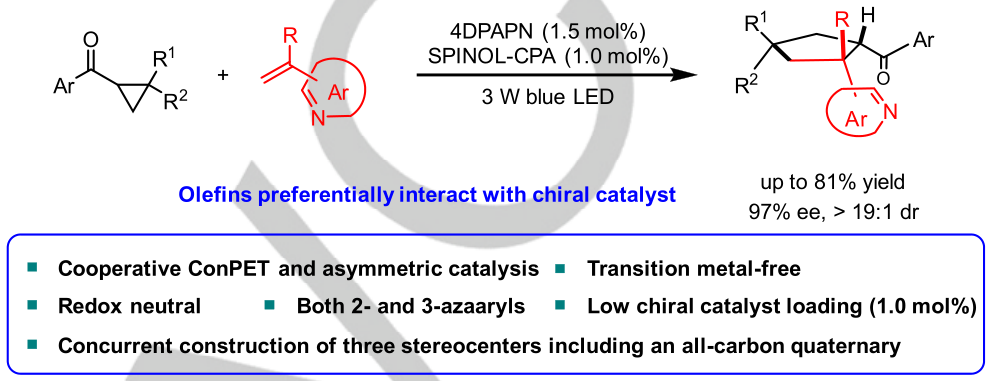
134. Wei, W., Li, C., Fan, Y., Chen, X., Zhao, X., Qiao, B.*, Jiang, Z.*, Catalytic Asymmetric Redox-Neutral [3 + 2] Photocycloadditions of Cyclopropyl Ketones with Vinylazaarenes Enabled by Consecutive Photoinduced Electron Transfer. Angew. Chem. Int. Ed. 2024, e202406845.
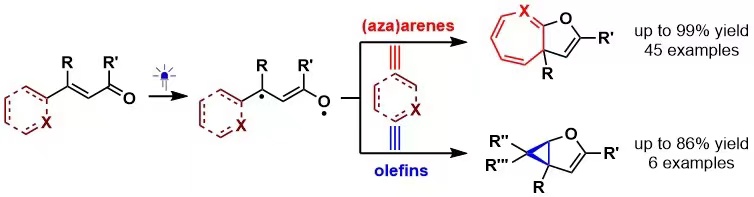
133. Zhang, L., You, M., Ban, X., Zhao, X., Yin, Y., Cao, S.*, Jiang, Z.*, Visible Light-Driven Dearomative Ring Expansion of (Aza)arenes to Access Dihydrofuran-based Polycyclic Compounds. Chem. Sci. 2024, 15, 8828-8834.
132. Ban, X.*, Jiang, Z.*, Asymmetric Photochemical Transformations Using a Chiral Hydrogen Bond Donor. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. 2024, DOI:org/10.1016/B978-0-32-390644-9.00135-9
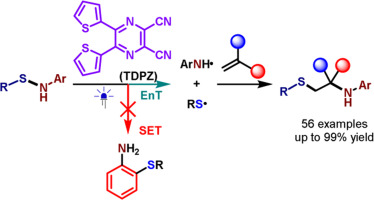
131. Wang, E.-M., Wang, Z., Ban, X., Zhao, X., Yin, Y.*, Jiang, Z.*, Chemoselective Photocatalytic Sulfenylamination of Alkenes with Sulfenamides via Energy Transfer. Chin. Chem. Lett. 2024, DOI:org/10.1016/j.cclet.2024.109843
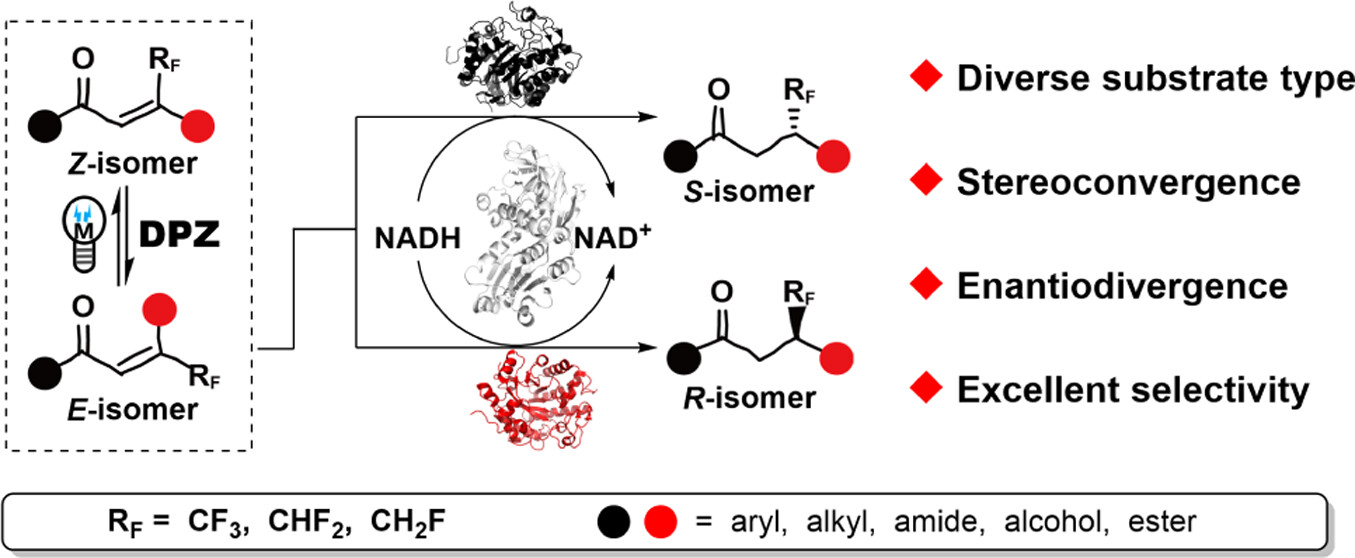
130. Zhang, L., Ma, J., Ban, X., Zhao, X., Yin, Y., Jiang, Z.*, Direct Enantioselective Reduction of C=C Bond of β-Polyfluoro-alkylated Enones via Asymmetric Photoredox Catalysis. Sci China Chem. 2024, 67, 2016-2021.
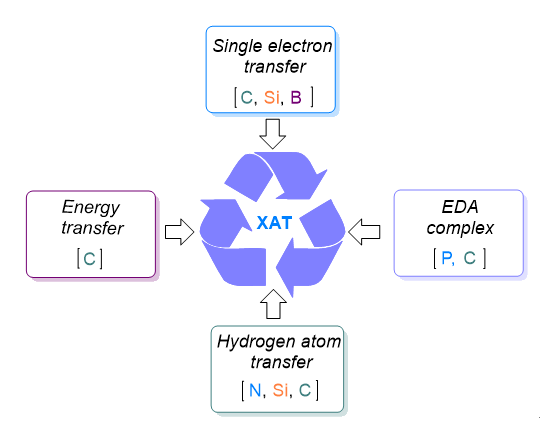
129. Jiang, Y., Yin, Y.*, Jiang, Z.* Recent Advances in Strategies for Halide Atom Transfer (XAT) and their Applications. Chinese J. Org. Chem. 2024, 44, 1733-1759.
2023
128. Ma, C., Shen, J., Qu, C., Shao, T.*, Cao, S.*, Yin, Y., Zhao, X., Jiang, Z.*, Enantioselective Chemodivergent Three-Component Radical Tandem Reactions through Asymmetric Photoredox Catalysis. J. Am. Chem. Soc.&